SORRY, SOLD OUT!
This discounted Applied Pharmacogenomics Program registration rate requires enrollee to have a paid full meeting registration for the 2018 ACCP Global Conference on Clinical Pharmacy in Seattle.
The ACCP Academy’s Certificate Program, Precision Medicine: Applied Pharmacogenomics is a practice-based activity designed to help clinical pharmacists understand how genetic factors influence the efficacy and adverse effects of drug therapy. Participants will learn empirical strategies for applying this knowledge in a clinical setting.
Target Audience
Clinical pharmacists seeking to understand the principles of pharmacogenomics, integrate precision medication services into their practice, and/or educate trainees, patients, and other health care professionals about pharmacogenomics.
Benefits
- Practice-based activity provides practical guidance for the application of pharmacogenomic principles in current patient populations.
- Experienced, engaging faculty deliver a proven curriculum in an accessible format.
- A combination of live and online modules encourage participants to interact with instructors and peers for an enhanced educational experience.
- Activities are evaluated throughout the program to reinforce the content and assess each participant’s application of the material.
- Twenty hours of programming across seven modules and twelve topics.
Learning Objectives
- Demonstrate an understanding of basic genetic concepts and nomenclature, and how genetic variation contributes to inter-individual variability in drug pharmacokinetics, pharmacodynamics, and adverse effects.
- Recommend clinical pharmacogenomic testing, when appropriate, and interpret the results to guide optimal drug selection and dosing.
- Use evidence-based guidelines and literature to formulate patient-specific medication recommendations based on pharmacogenomic test results and other patient-specific factors, and communicate these recommendations to the health care team.
- Educate patients and health care professionals about the principles of clinical pharmacogenomics, including clinical utility, cost-effectiveness, reimbursement, and ethical, legal, and social implications of pharmacogenomic testing.
- Develop a clinical pharmacogenomics implementation plan, including an assessment of clinical informatics resources (e.g., clinical decision support tools), which can be applied at the institutional or system level.
Technical Requirements
A computer with a high speed internet connection and audio is necessary. A webcam is preferred, but not required.
Our Committment to Privacy
Your privacy is important to us. To better protect your privacy, we provide a complete explanation of our online information practices and the choices you can make about the way your information is collected and used. Please visit https://www.accp.com/about/privacy.aspx to read our complete privacy statement.
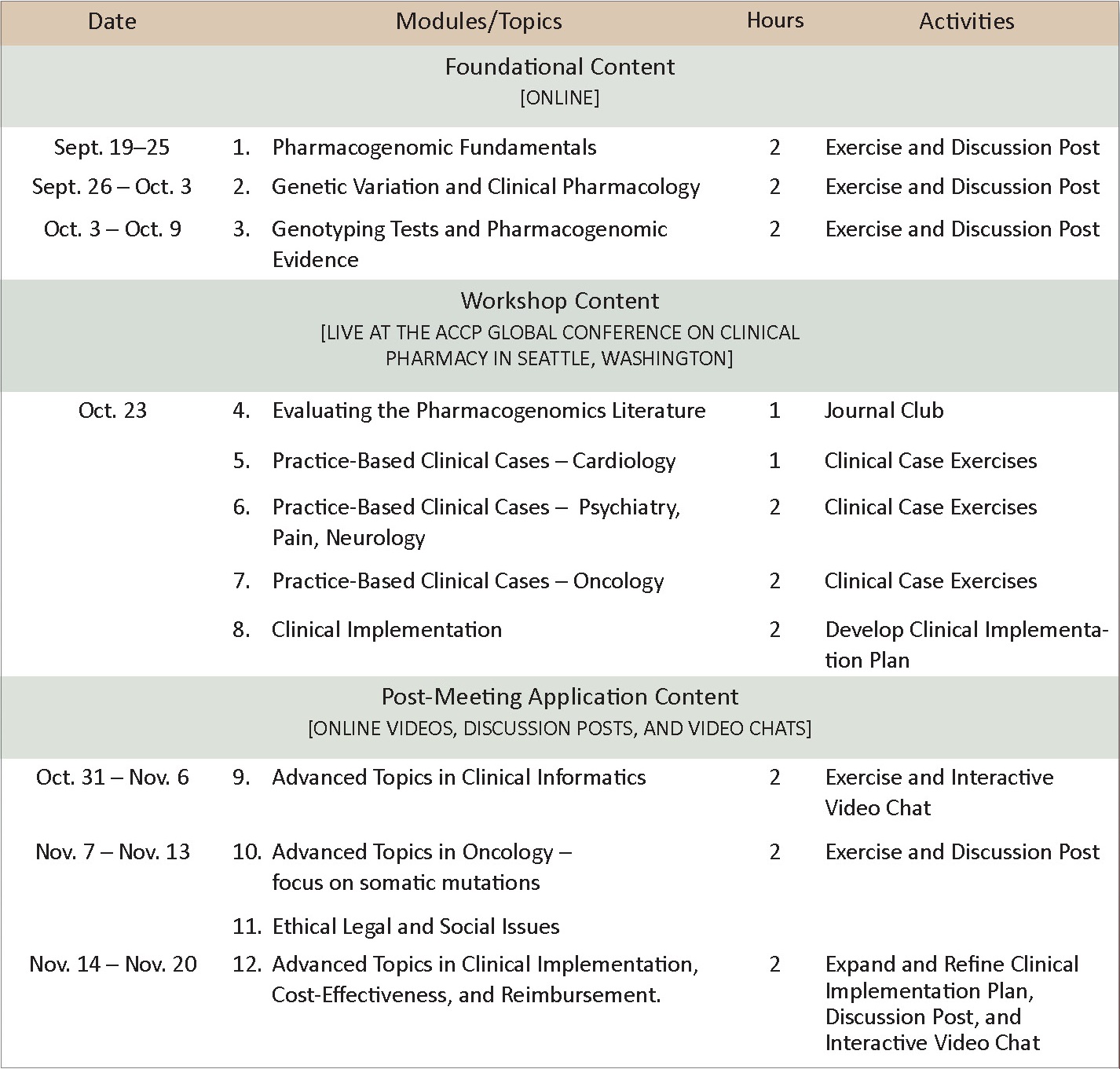
Schedule
Faculty
Christina L. Aquilante, Pharm.D.
Professor
Department of Pharmaceutical Sciences
Skaggs School of Pharmacy and Pharmaceutical Sciences
University of Colorado Denver
J. Kevin Hicks Pharm.D., Ph.D.
Personalized Medicine Specialist
Department of Individualized Cancer Management
Moffitt Cancer Center
Aniwaa Owusu Obeng, Pharm.D.
Assistant Professor
The Charles Bronfman Institute for Personalized Medicine
Icahn School of Medicine at Mount Sinai
Drs. Aquilante, Hicks and Obeng have no relevant financial relationships to disclose.
 The American College of Clinical Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. The Universal Activity Number (UAN) for this activity is UAN:0217-0000-18-224-B04-P
The American College of Clinical Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. The Universal Activity Number (UAN) for this activity is UAN:0217-0000-18-224-B04-P
Successful completion of all of the requisites, no later than December 15, 2018, will provide 20.0 contact hours (2.0 CEU) of practice-based continuing pharmacy education (10 hours home-study, 10 hours live) and a certificate suitable for framing. To achieve the stated program outcomes, the activity is designed to be completed in its entirety. No continuing education credit will be given for partial completion of the program. Program completion and participant information will be submitted online via CPE Monitor®.